Low Dose Sorafenib in Gastric Gastrointestinal Stromal Tumour with PDGFRA p.1843-D846 Deletion in an 88-Year-Old Male
By Asif Husain OsmaniAffiliations
doi: 10.29271/jcpsp.2024.04.505Sir,
Gastrointestinal stromal tumours (GISTs) are mesenchymal tumours of the gastrointestinal tract (GIT) usually diagnosed at the mean age of 65 years. However, 20% or more are diagnosed over 70 years of age.1 Thorough understanding of molecular biology plays a pivotal role in its management, especially in elderly population where both effectiveness and tolerability of systemic therapy are considered crucial. We present a case of an elderly male with gastric GIST exhibiting a clinically aggressive course.
An 88-year-old male, ex-smoker, presented with complaints of epigastric pain and vomiting. Initial evaluation in the Emergency Room by ultrasound abdomen revealed a large thick walled heterogeneous area identified at the left lumbar region at the site of pain and swelling. It measured 15.5 × 12.3 cm and showed diffuse low-level moving internal echoes. CT scan abdomen revealed a well circumscribed predominantly cystic lesion arising from the posterior wall of the stomach. It measured approximately 129 × 164 × 182 mm in AP, TS, and CC dimensions, respectively. On post-contrast images, it showed peri-pheral enhancement with enhancing solid components and mural nodules attached to the wall of the cystic lesion. Anterolaterally, it was closely abutting the anterolateral abdominal wall with slightly indistinct fat planes. It was displacing the adjacent small bowel loops. Posteriorly, it was also closely abutting the body and tail of the pancreas without definite evidence of infiltration. Fat stranding was identified surrounding this lesion (Figure 1). He underwent a CT-guided biopsy of the mass. Histopathology revealed a neoplasm composed of spindle-shaped cells loosely arranged in bundles and fascicles with areas of necrosis. Immunohistochemical (IHC) panel showed CD117, DOG-1, CD99, FLI-1, and desmin positivity in spindle cells, whereas CD34, CD31, SMA, caldesmon, myogenin, MYO-D1, CKAE1/AE3, CKLMW, CKHMW, S-100, SOX-10, synaptophysin, STAT-6, B-catenin, HMB-45, Mart-1, and EMA were negative. Furthermore, mutational analysis was consistent with Micro-satellite Stable (MSI-S), Tumour mutational burden- 1 mut/Mb, PDGFRA p.1843-D846 deletion, and CDKN2A/B rearrangement. Cyst fluid cytology was negative for malignancy. Later, a PET-CT scan revealed a large cystic mass lesion arising from the submucosa of the posterior wall of the stomach with extension to the lesser sac and abutting the pancreas, adjacent gut loops and the abdominal wall with SUVmax of 8.8 and it measured 13.3 × 21.4 × 26 cm in size. Multiple partially calcified lymph nodes were noted in the mediastinum and right para tracheal, pre-aortic, aorto-pulmonary, pre-carinal, bilateral hilar, and sub-carinal regions. SUVmax of these lymph nodes ranged up to 6.6 and majority of them were subcentimeter in size.
In two months, he presented to the hospital with progressive abdominal distention and had undergone ultrasound-guided fluid drainage from cystic mass more than 10 times with quantity of fluid ranging from 450 to 2700 ml per shift. During this period, he was commenced on Imatinib, 400 mg, once daily. The frequency and amount of cyst fluid drainage declined steadily while on imatinib therapy. However, after 2 months of therapy, the disease started progressing and repeat imaging revealed a well-defined heterogeneous mass with predominantly cystic component giving no definite internal vascularity on colour Doppler imaging, mostly in the left hypochondrium and now measuring 21.2 × 19.4 × 21.8 cm (Figure 2). He was switched to Sorafenib, 200 mg once daily, due to disease progression and further decline in his overall general condition. Four months after the initiation of Sorafenib, the gastric mass showed a clinical response, and the drug was well tolerated without any adverse effects. However, during the fifth month, the patient died due to aspiration pneumonia.
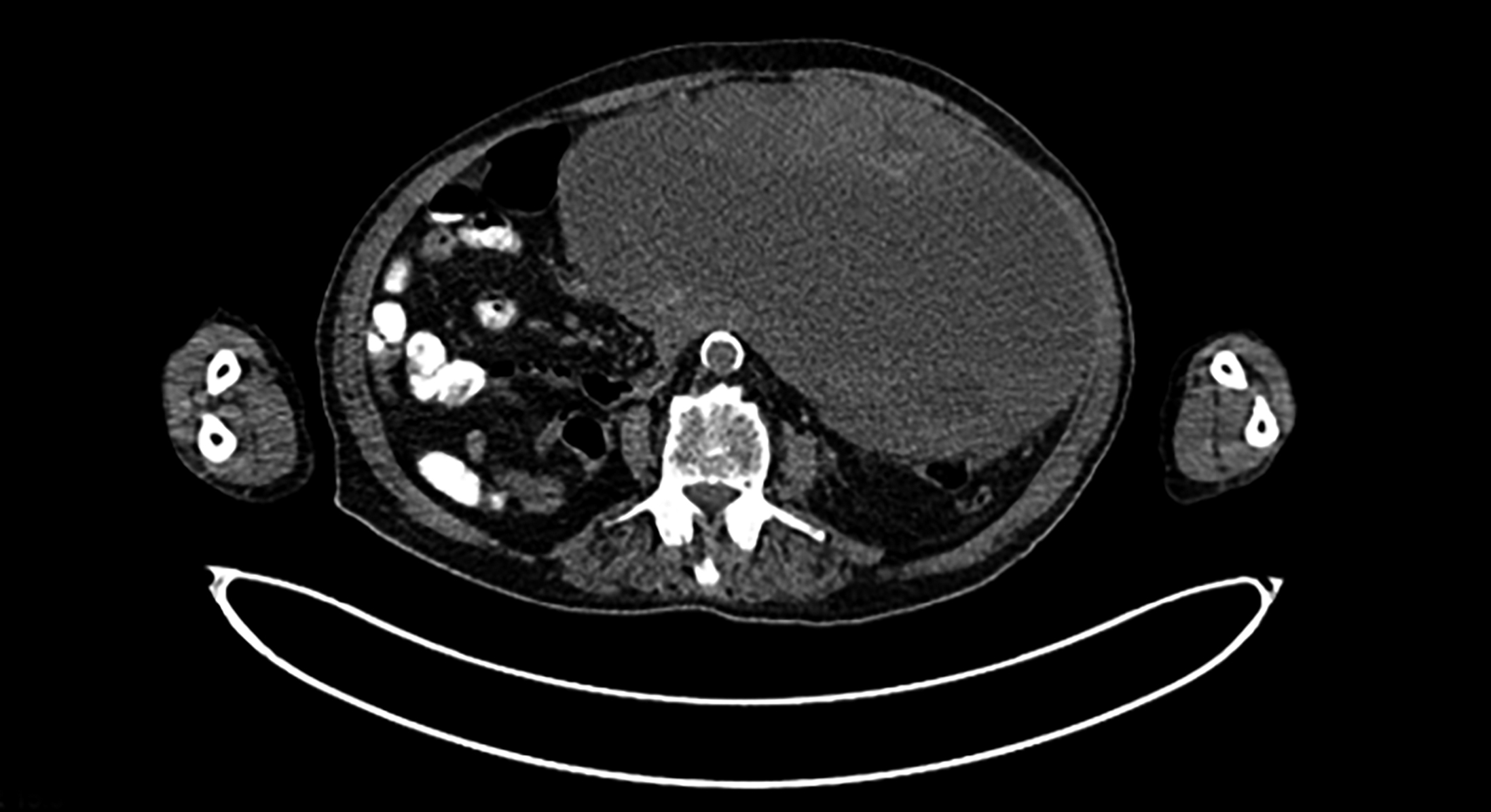 Figure 1: CT abdomen. A well circumscribed predominantly cystic lesion is seen arising from the posterior wall of the stomach. It measures approximately 12.9×16.4 × 18.2 cm in AP, TS, and CC dimensions. On post-contrast images, it shows peripheral enhancement with enhancing solid component and mural nodules attached to the wall of cystic lesion.
Figure 1: CT abdomen. A well circumscribed predominantly cystic lesion is seen arising from the posterior wall of the stomach. It measures approximately 12.9×16.4 × 18.2 cm in AP, TS, and CC dimensions. On post-contrast images, it shows peripheral enhancement with enhancing solid component and mural nodules attached to the wall of cystic lesion.
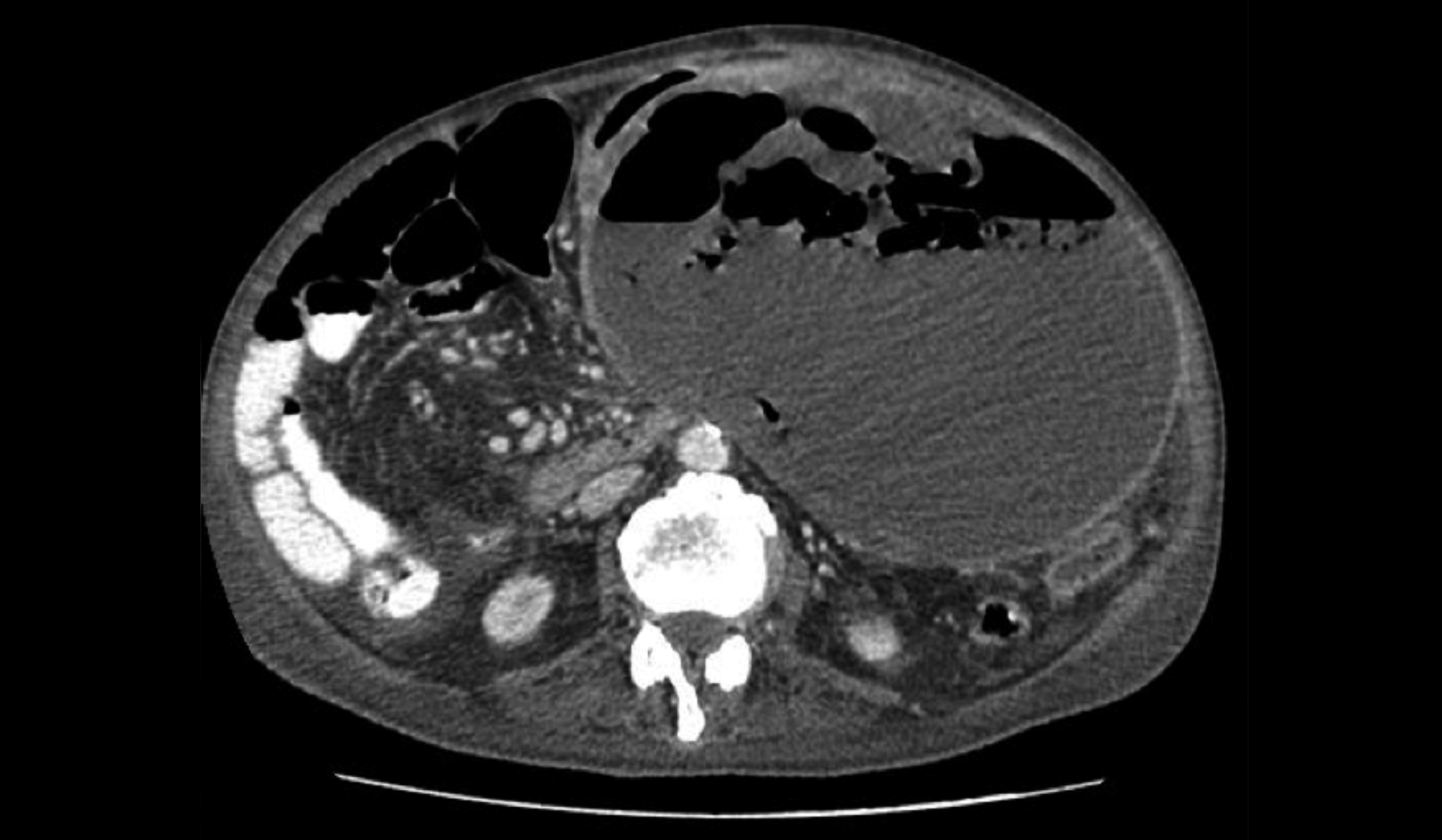 Figure 2: CT abdomen. A large cystic lesion with peripheral nodular enhancement identified arising from posterior wall of stomach extending to left hypochondrium up to the pelvis.
Figure 2: CT abdomen. A large cystic lesion with peripheral nodular enhancement identified arising from posterior wall of stomach extending to left hypochondrium up to the pelvis.
Imatinib is frequently used in GISTs, both in perioperative and metastatic settings, requiring dose adjustments due to toxicity, and should be carefully considered in the elderly population. It is considered as initial line of treatment for GIST that inhibits a few kinases that include PDGFRα and protein KIT.2 Among all aberrations of PDGFRα, substitution at codon D842 located at exon 18 constitutes 63% while the deletions p.D842-H845 (DIMH842- 845) and p.I843-D846 (IMH843-846) account for 15%.3 A phase II study investigated Sorafenib as the third-line treatment in advanced GISTs reporting median progression-free survival (PFS) of 4.9 months regardless of mutational status.4 Brinch et al, reported an outstanding response of Sorafenib with deletion of codon p.I843-D846del in recurrent GIST.5
COMPETING INTEREST:
The author declared no conflict of interest.
AUTHOR’S CONTRIBUTION:
AHO: Conceived the idea, literature search, and drafted the manuscript. The author approved the final version of the manuscript to be published.
REFERENCES
- Joensuu H. Gastrointestinal stromal tumor (GIST). Ann Oncol 2006; 17 (Suppl 10):280-6. doi: 10.1093/annonc/ mdl274.
- Bauer S, Joensuu H. Emerging agents for the treatment of advanced, imatinib-resistant gastrointestinal stromal tumors: Current status and future directions. Drugs 2015; 75(12):1323-34. doi: 10.1007/s40265-015-0440-8.
- Corless CL, Schroeder A, Griffith D, Town A, McGreevey L, Harrell P, et al. PDGFRA mutations in gastrointestinal stromal tumors: Frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol 2005; 23(23):5357-64. doi: 10.1200/JCO.2005.14.068.
- Park SH, Ryu MH, Ryoo BY, Im SA, Kwon HC, Lee SS, et al. Sorafenib in patients with metastatic gastrointestinal stromal tumors who failed two or more prior tyrosine kinase inhibitors: A phase II study of Korean gastro-intestinal stromal tumors study group. Invest New Drugs 2012; 30(6):2377-83. doi: 10.1007/s10637-012-9795-9.
- Brinch C, Dehnfeld M, Hogdall E, Poulsen TS, Toxvaerd A, Al-Farra G, et al. Outstanding response to sorafenib in a patient with metastatic gastrointestinal stromal tumour. Case Rep Oncol 2021; 14(3):1567-73. doi: 10.1159/000 519747.